Extended Release System
Welke werkingsmechanismen zijn er beschikbaar om een continue en betrouwbare afgifte van een geneesmiddel te garanderen voor een periode van 6 maanden? Lees hier de uitleg over een van deze mechanismen: de Atrigel®-techniek van Eligard®.
ATRIGEL® Delivery System: Drug Release Mechanism1
ELIGARD® is a mixture of the peptide LA (leuprorelin acetate), biodegradeable polymers (lactide and glycolide polymers) and biocompatible carrier (solvent). When the liquid hits the subcutaneous space, an exchange between the water and the organic carrier/solvent occurs, leading to a phase inversion and precipitation of the polymer, forming a solid which traps the LA into the in-situ solid implant controlling the rate of drug release.
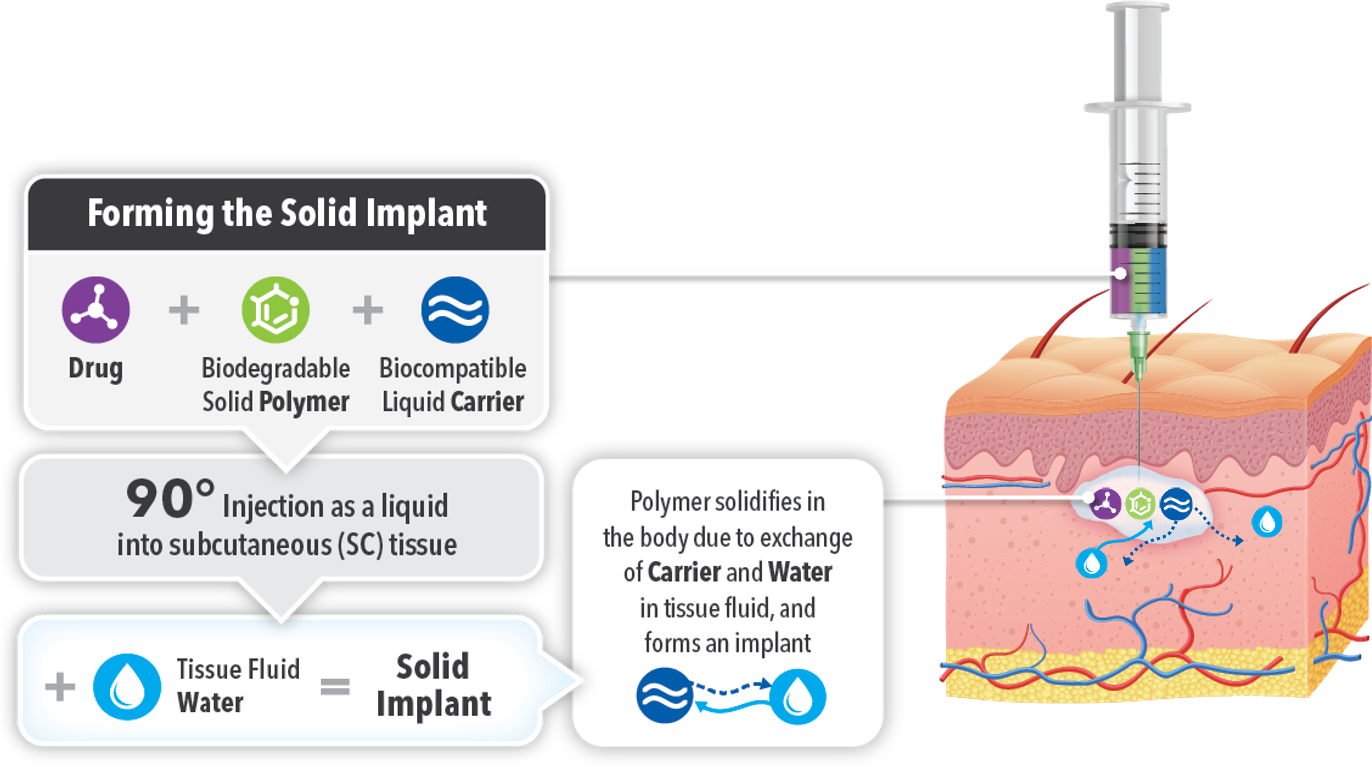
(Adapted from: Sartor O. Eur. Urol. 2006; SmPC ELigard 09-2022; Parent M. et al. J Controlled Release 2013: 172: 292-304.)
The biodegradable, water insoluble polymers degrade into lactic and glycolytic acids by hydrolysis, releasing the drug. These acids are eliminated as CO2 and water into the body by the tricarboxylic acid cycle. Over time you see the drug continuing to be released in a controlled fashion, while the natural byproducts (CO2 and H2O) are eliminated safely.
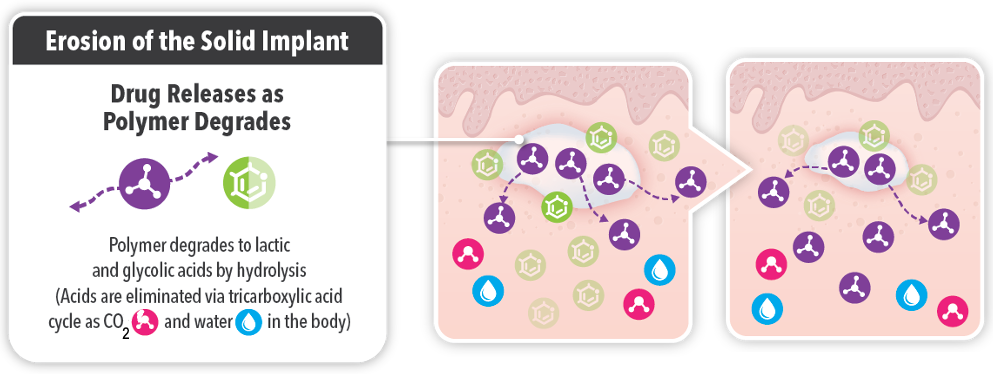
(Adapted from: Sartor O. Eur. Urol. 2006; SmPC ELigard 09-2022; Parent M. et al. J Controlled Release 2013: 172: 292-304.)
ELIGARD® In-Situ Implant Composition
The ratio of the dual-polymers in ATRIGEL® was optimized for each dosing period. Formulation optimization also enables the volume of drug to be kept small.
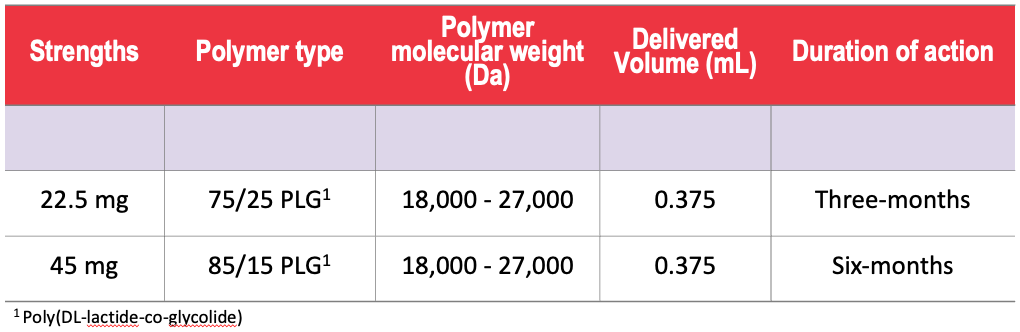
(Adapted from: Prettyman, et at. Urologic Nursing. 2019)
Referentie
1 Sartor O. Eur. Urol. 2006
Deze pagina is mogelijk gemaakt door:
